An example of such a spectrum is that of the flavoring agent vanillin shown below.
Ir spectroscopy ch vinylic.
Infrared spectroscopy ir spectroscopy or vibrational spectroscopy is the measurement of the interaction of infrared radiation with matter by absorption emission or reflection it is used to study and identify chemical substances or functional groups in solid liquid or gaseous forms.
The vinylic c h bond expected to be 3100 cm 1 3 all other c h bonds.
Look for double bonds 1600 1850cm 1 2.
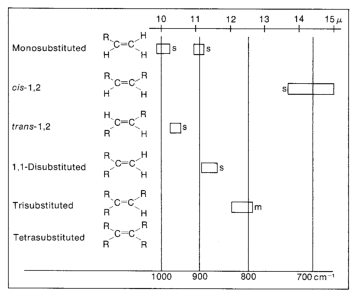
Use the examples linked to the table to see the profile and intensity of bands.
Steps to analyzing an ir spectrum.
847 853 look over pages 853 866 after viewing this presentation for additional examples of various functional groups.
Vinyl alcohol ir spectra.
Emphasis is on data interpretation.
Each of the sentences below appears verbatim in the section.
Nitrogen is connected to 1 carbon produces two signals one at 3350 cm 1 and the other 3450 cm 1.
The ir spectrum table is a chart for use during infrared spectroscopy the table lists ir spectroscopy frequency ranges appearance of the vibration and absorptions for functional groups.
833 834 in lab textbook uses of the infrared spectrum p.
There are two tables grouped by frequency range and compound class.
You can locate carbonyl groups alkenes alkynes and aromatics in the ir infrared spectrum based on their shapes and relative locations.
Inductive pull of cl increases the electron density between c and o.
Acyl all ir values are approximate and have a range of possibilities depending on the molecular environment in which the functional group resides.
To verify that your answers are correct look in your textbook at the end of chapter 15.
Be careful to avoid focusing on selected absorption bands and overlooking others.
The method or technique of infrared spectroscopy is conducted with an instrument called an infrared.
Infrared spectroscopy ir theory and interpretation of ir spectra assigned readings introduction to technique 25 p.
Infrared spectrometers similar in principle to the uv visible spectrometer described elsewhere permit chemists to obtain absorption spectra of compounds that are a unique reflection of their molecular structure.
For one thing all of these functional groups appear to the right of the c h absorptions which always occur between 2 800 cm 1 to 3 000 cm 1 in the ir spectrum and to the left of.
Infrared spectroscopy and mass spectrometry review of concepts fill in the blanks below.
.pdf+-+SumatraPDF_2012-12-08_21-20-25.png)
.pdf+-+SumatraPDF_2012-12-08_21-20-25.png)